Palladium-catalyzed dicarbonylative synthesis of tetracycle quinazolinones†
Abstract
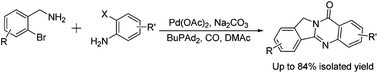
An interesting procedure for the synthesis of isoindolo[1,2-b]quinazolin-10(12H)-ones has been developed. Starting from commercially available 2-bromoanilines and 2-bromobenzyl amines, with the assistance of a palladium catalyst, the desired products were isolated in good yields. Notably, this procedure proceeded in a highly selective manner; two molecules of CO were incorporated into the substrates selectively.

 Please wait while we load your content...
Please wait while we load your content...