σ-Hole⋯π and lone pair⋯π interactions in benzylic halides†
Abstract
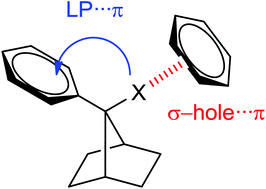
Intermolecular and intramolecular halogen⋯π interactions in benzylic halides (Ph–CR2–X; X = F, Cl, Br and I) derived from 7-phenylnorbornane were investigated. The imposed geometry of the 7-arylnorbornane moiety prevents the participation of intramolecular attractive interactions between the σ-hole region of the halogen atom and the π electrons of the aromatic ring. Crystallographic data show intermolecular halogen bonds in iodide 1 and bromide 2 in the solid state. On the other hand, both UV-Vis and D-NMR data suggest the occurrence of intramolecular interactions between the halogen atoms and the phenyl rings in these compounds in solution. To provide more insight into the nature of the observed stabilizing interactions, density functional calculations were also carried out. These computations confirm the presence of genuine lone pair⋯π intramolecular interactions which strongly affect the stability and the electronic structure of these species.

 Please wait while we load your content...
Please wait while we load your content...