Chirality extension of an oxazine building block en route to total syntheses of (+)-hyacinthacine A2 and sphingofungin B†
Abstract
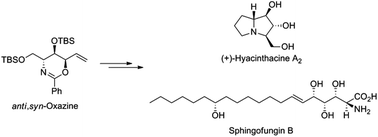
Concise and stereocontrolled syntheses of (+)-hyacinthacine A2 and sphingofungin B were achieved via a diastereomerically enriched oxazine intermediate. The key strategies include palladium(0)-catalyzed intramolecular oxazine formation and diastereoselective nucleophilic addition to an aldehyde. (+)-Hyacinthacine A2 was synthesized in 13 steps and 10.2% overall yield and the synthesis of sphingofungin B proceeded in a linear sequence over 15 steps and 6.9% overall yield from (R)-methyl 2-benzamido-3-((tert-butyldimethylsilyl)oxy)propanoate.

 Please wait while we load your content...
Please wait while we load your content...