Semi-synthesis of thioamide containing proteins
Abstract
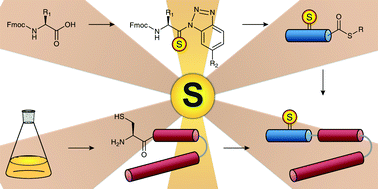
Our laboratory has shown that the thioamide, a single atom O-to-S substitution, can be a versatile fluorescence quenching probe that is minimally-perturbing when placed at many locations in a protein sequence. In order to make these and other thioamide experiments applicable to full-sized proteins, we have developed methods for incorporating thioamides by generating thiopeptide fragments through solid phase synthesis and ligating them to protein fragments expressed in E. coli. To install donor fluorophores, we have adapted unnatural amino acid mutagenesis methods, including the generation of new tRNA synthetases for the incorporation of small, intrinsically fluorescent amino acids. We have used a combination of these two methods, as well as chemoenzymatic protein modification, to efficiently install sidechain and backbone modifications to generate proteins labeled with fluorophore/thioamide pairs.

 Please wait while we load your content...
Please wait while we load your content...