DMSO/Tf2O-mediated cross-coupling of tryptamine with substituted aniline to access C3a–N1′-linked pyrroloindoline alkaloids†
Abstract
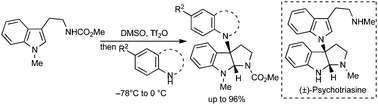
The cross-coupling of tryptamine with substituted aniline to access C3a–nitrogen-linked pyrroloindolines has been developed via the consecutive cyclization of tryptamine with DMSO/Tf2O and the substitution of 3a-pyrroloindolylthionium intermediate with aniline. The use of 2,3-dihydrotryptamine instead of aniline enabled easy access to 3a-(1-indolyl)pyrroloindoline and the concise synthesis of C3a–N1′-linked pyrroloindoline alkaloid (±)-psychotriasine was accomplished.

 Please wait while we load your content...
Please wait while we load your content...