Direct biosynthetic cyclization of a distorted paracyclophane highlighted by double isotopic labelling of l-tyrosine†
Abstract
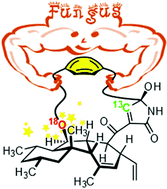
The biosynthesis of pyrrocidines, fungal PK-NRP compounds featuring a strained [9]paracyclophane, was investigated in Acremonium zeae. We used a synthetic L-tyrosine probe, labelled with oxygen 18 as a reporter of phenol reactivity and carbon 13 as a tracer of incorporation of this exogenous precursor. The (18O)phenolic oxygen was incorporated, suggesting that phenol behaves as a nucleophile during the formation of the bent aryl ether.


 Please wait while we load your content...
Please wait while we load your content...