A modular synthesis of functionalised phenols enabled by controlled boron speciation†
Abstract
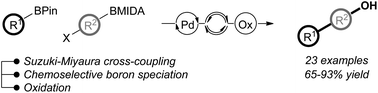
A modular synthesis of functionalised biaryl phenols from two boronic acid derivatives has been developed via one-pot Suzuki–Miyaura cross-coupling, chemoselective control of boron solution speciation to generate a reactive boronic ester in situ, and oxidation. The utility of this method has been further demonstrated by application in the synthesis of drug molecules and components of organic electronics, as well as within iterative cross-coupling.

 Please wait while we load your content...
Please wait while we load your content...