First total synthesis of the marine natural products clavulolactones II and III†
Abstract
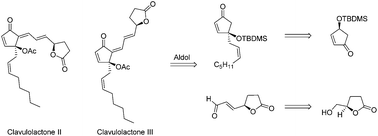
The first total synthesis of the marine prostanoids clavulolactones II and III is presented from an easily accessible chiral, non-racemic cyclopentenone intermediate. Key steps involve selective TBDMS deprotection, selective reduction of the β-side chain and aldol condensation. Clavulolactones II and III were successfully prepared from (S)-4-((tert-butyldimethylsilyl)oxy) cyclopent-2-en-1-one over nine steps, in overall yields of 21 and 7% respectively.

 Please wait while we load your content...
Please wait while we load your content...