γ-Aminoalcohol rearrangement applied to pentahydroxylated azepanes provides pyrrolidines epimeric to homoDMDP†
Abstract
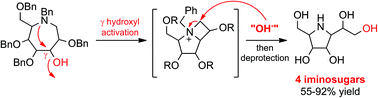
A series of pentahydroxylated pyrrolidines, displaying five contiguous stereogenic centres and epimeric to α-glucosidase inhibitor homoDMDP, have been synthesized. The key step involves a γ-aminoalcohol rearrangement applied to polyhydroxylated azepanes. These five-membered iminosugars demonstrate micromolar inhibition of glycosidases.

 Please wait while we load your content...
Please wait while we load your content...