An efficient approach to gem-difluorocyclopropylstannanes via highly regio- and stereoselective hydrostannylation of gem-difluorocyclopropenes and their unique ring-opening reaction to afford β-fluoroallylic alcohols†
Abstract
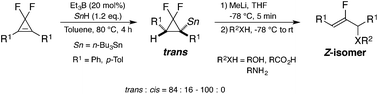
Treatment of various gem-difluorocyclopropenes with 1.2 equiv. of n-Bu3SnH in the presence of 20 mol% of Et3B at 80 °C for 4 h led to the quantitative formation of the hydrostannylated products in a highly regio- and trans-selective manner. Additionally, the prepared trans-gem-difluorocyclopropylstannanes were treated with 1.5 equiv. of MeLi in THF at −78 °C for 5 min, followed by quenching the reaction with various agents, such as H2O, alcohols, carboxylic acids, and tosylamide, to give the corresponding β-fluoroallylic alcohols, ethers, esters, and amides respectively with exclusive Z selectivity in acceptable yields.

 Please wait while we load your content...
Please wait while we load your content...