C-terminal heat shock protein 90 modulators produce desirable oncogenic properties
Abstract
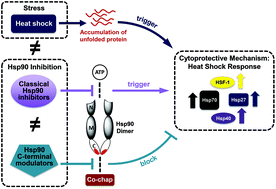
The cellular protection mechanism, the heat shock response, is only activated by classical heat shock 90 inhibitors (Hsp90) that “target” the N-terminus of the protein, but not by those that modulate the C-terminus. Significant differences in cytotoxicity (nanomolar) for classical inhibitors versus their ability to modulate Hsp90 (low micromolar) are discussed. In contrast, molecules that modulate Hsp90's C-terminus show similar IC50 values for cytotoxicity and Hsp90 inhibition. A comparison between the two types of Hsp90 inhibitors suggests that classical inhibitors may be modulating an alternative biological target that stresses the cell rather directly inhibiting Hsp90, whereas C-terminal modulators are most likely acting by directly inhibiting Hsp90.

 Please wait while we load your content...
Please wait while we load your content...