3-Nitro-2-pyridinesulfenyl-mediated solid-phase disulfide ligation in the synthesis of disulfide bond-containing cyclic peptides†
Abstract
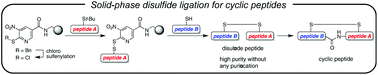
A new solid-phase disulfide ligation method is developed to prepare a disulfide peptide from two types of Cys-containing peptide fragments with minimum purification steps. In combination with the subsequent intramolecular amide bond formation, a cyclic nonapeptide, oxytocin, was efficiently synthesized as a fundamental model for more complex cyclic peptides.

 Please wait while we load your content...
Please wait while we load your content...