One-pot total synthesis of streptindole, arsindoline B and their congeners through tandem decarboxylative deaminative dual-coupling reaction of amino acids with indoles†
Abstract
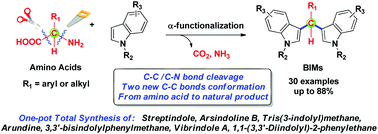
This paper described a decarboxylative deaminative dual-coupling reaction of amino acids with indoles to afford BIM scaffolds and its further application to the one-pot total synthesis of natural products. This method featured a stimulating example of activating amino acids in one pot as multi-carbon building blocks for transformation into final targets which are equipped with amino acid side chain backbones.

 Please wait while we load your content...
Please wait while we load your content...