Design and solid phase synthesis of new DOTA conjugated (+)-biotin dimers planned to develop molecular weight-tuned avidin oligomers†
Abstract
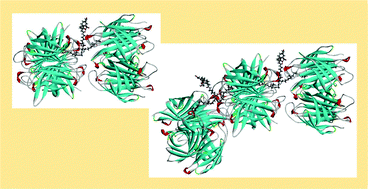
Chemical modifications of the biotin carrier in pretargeted avidin–biotin radionuclide therapy may be of paramount importance for tuning the amount of the radioactivity delivered to cancer cells by labelled biotins. We report here the synthesis of a collection of new synthetic DOTA-constructs bearing two (+)-biotin molecules (bis-biotins), designed for the creation of multimeric Av units (tetramers) bonded to the antibody. All the syntheses were carried out following the solid phase strategy and growing the molecules on a Rink Amide resin. The biotin heads are connected through spacers containing PEG or non-PEG residues. Molecular modelling calculations suggested that the Av cross-linking ability of the bis-biotins depends mainly on the spacers length, with the best results being expected for arms affording distances in the range of 10–25 Å between the biotin carboxylate atoms, in the fully extended conformation. SEC-HPLC MALLS analysis of the products of our Av/bis-biotin reaction mixtures have confirmed this hypothesis. The bis-biotin 16, where the non-PEG linker ensured a distance of 26.7 Å between the biotin moieties, gave about 50% of Av oligomers while the shorter analogue 18 (19.5 Å) afforded 100% of an Av polymer containing about 21 protein units. Remarkably, the solubility of both the bis-biotins, i.e.16 and 18, in aqueous solutions was good and they showed excellent stability against the action of peptidases.

 Please wait while we load your content...
Please wait while we load your content...