Total syntheses of five uvacalols: structural validation of uvacalol A, uvacalol B and uvacalol C and disproval of the structures of uvacalol E and uvacalol G†
Abstract
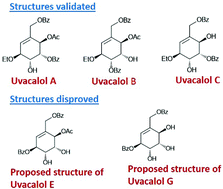
Uvacalols are novel carbasugars belonging to the family of C7-cyclitols, and are isolated from the roots of the medicinal plant, Uvaria calamistrata. In this study, we report the first syntheses of five uvacalols starting from a cheap and easily available chiral pool starting material, D-mannitol, in their optically pure form. D-Mannitol was converted to the alkene 2 through a series of regioselective and chemoselective transformations by following our previously reported strategies. Alkene 2 was converted to the enal 5 through a series of protective group manipulations. Enal 5 was converted to the diene 6 by the addition of vinyl magnesium bromide. Ring closing metathesis of the diene 6 using Grubbs’ second generation catalyst installed the core cyclohexenyl unit. Through several iterative and selective manipulations of various hydroxyl groups, uvacalol A, uvacalol B, uvacalol C, uvacalol E and uvacalol G were synthesized. A comparison of the 1H NMR and 13C NMR data of these synthesized molecules with the reported data, revealed that the reported structures of uvacalols A–C are correct and those of uvacalols E and G are wrong.

 Please wait while we load your content...
Please wait while we load your content...