Synthesis and assessment of 4-aminotetrahydroquinazoline derivatives as tick-borne encephalitis virus reproduction inhibitors†
Abstract
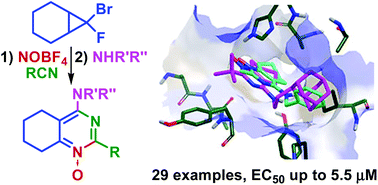
Tick-borne encephalitis virus (TBEV) belonging to Flavivirus genus causes severe infection in humans. The search for therapeutically relevant compounds targeting TBEV requires the exploration of novel chemotypes. A versatile synthesis of previously unknown 4-aminopyrimidines and 4-aminopyrimidine N-oxides based on a fluorosubstituted heterocyclic core is described. A representative series of 4-aminotetrahydroquinazoline derivatives, containing aliphatic and aromatic substituents as well as the adamantane framework, was obtained and their activity against tick-borne encephalitis virus reproduction was studied. Nine compounds were found to inhibit TBEV entry into the host cells. A bulky hydrophobic adamantyl group was identified to be important for the antiviral activity. The developed synthetic route allowed an easy access to a consistent compound library for further structure–activity relationship studies.


 Please wait while we load your content...
Please wait while we load your content...