Luminescent organogels based on triphenylamine functionalized β-diketones and their difluoroboron complexes†
Abstract
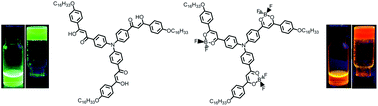
A series of new triphenylamine functionalized β-diketones 1–3 and their difluoroboron complexes 1B–3B were synthesized. They exhibited strong intramolecular charge transfer (ICT) emission. It was found that their self-assembling properties depended on the molecular structures. For example, compounds 1 and 1B, in which only one β-diketone or difluoroboron β-diketone unit was linked to triphenylamine, showed better gelation abilities directed by π–π interaction. Although bis-β-diketone substituted triphenylamine 2 could not form organogels, its difluoroboron complex 2B could gel DMSO due to the strong dipole–dipole interactions. Compound 3 could form gels in polar solvents, while 3B formed gels in nonpolar solvents. Notably, the asymmetric gelators 1, 1B and 2B exhibited AIEE behaviors during the gelation. Although the emission of the symmetric compounds 3 and 3B decreased to a certain degree upon gelation, the obtained gels still gave strong emission. The gels formed from 1 and 3 emitted strong green light and those based on 1B–3B emitted strong orange or red light. These highly luminescent materials might have potential applications in emitting devices and fluorescent sensors.

 Please wait while we load your content...
Please wait while we load your content...