Oxidative asymmetric umpolung alkylation of Evans’ β-ketoimides using dialkylzinc nucleophiles†
Abstract
Umpolung alkylation of Evans’ auxiliary substituted β-ketoimides affords the diastereomerically pure products in yields ranging from 40 to 80%. The reaction itself proceeds with diastereoselectivities between 3 : 1 and 18 : 1. Dialkylzinc serves as the nucleophile and umpolung of the β-keto-imide enolate is achieved by the action of Koser's reagent.
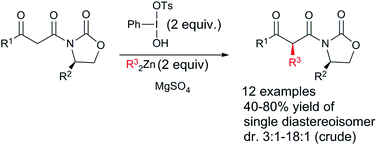

 Please wait while we load your content...
Please wait while we load your content...