An IDB-containing low molecular weight short peptide as an efficient DNA cleavage reagent†
Abstract
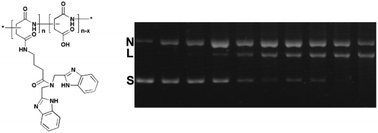
Artificial nucleases have attracted significant interest due to their abilities in accelerating DNA cleavage, which results in the possibility of genome manipulation. However, compared with natural nucleases, the currently available artificial nucleases have low cleavage efficiency, especially metal-free artificial nucleases. Thus, it is still a challenge to develop highly efficient metal-free artificial nucleases via a non-oxidative pathway. We here designed and prepared a group of rigid bis-amine-grafted PASP conjugates (PASP-IDB), and investigated their abilities to induce DNA double-strand cleavage. The detailed assays showed that in the absence of metal ions, these short peptide conjugates can effectively break the phosphodiester linkage at a relatively low concentration and under physiological conditions through a hydrolytic process, giving the 107-fold rate acceleration over uncatalyzed double-strand DNA. The probable mechanism verified by control experiments revealed that IDBs and free carboxyl groups in PASP synergically catalyzed DNA cleavage. In addition, the effects of degrees of substitution on the cleavage activity were studied, and the results indicated the existence of minimum building blocks of PASP-IDB for efficient DNA cleavage. The results of our study have implications on the design of short peptide-based molecules as new artificial nucleases and may provide a strategy for developing safe and efficient metal-free DNA cleavage reagents.

 Please wait while we load your content...
Please wait while we load your content...