Molecular characterization of the boron adducts of the proteasome inhibitor bortezomib with epigallocatechin-3-gallate and related polyphenols
Abstract
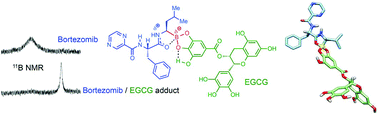
The green tea polyphenol epigallocatechin-3-gallate (EGCG) was reported to effectively antagonize the ability of Bortezomib (BZM) to induce apoptosis in cancer cells. This interaction was attributed to the formation of a covalent adduct between a phenolic moiety of EGCG with the boronic acid group of Bortezomib. However, the structural details of this boron adduct and the molecular factors that contribute to its formation and its ability to inhibit Bortezomib's activity remain unclear. This paper describes the use of NMR spectroscopy and cell assays to characterize the structures and properties of the boron adducts of EGCG and related polyphenols. The observed boron adducts included both boronate and borate derivatives, and their structural characteristics were correlated with cell-based evaluation of the ability of EGCG and other phenols to antagonize the anticancer activity of Bortezomib. The enhanced stability of the BZM/EGCG adduct was attributed to electronic and steric reasons, and a newly identified intramolecular interaction of the boron atom of BZM with the adjacent amide bond. The reported approach provides a useful method for determining the potential ability of polyphenols to form undesired adducts with boron-based drugs and interfere with their actions.

 Please wait while we load your content...
Please wait while we load your content...