Copper-catalyzed ring expansion of 2-aminobenzothiazoles with alkynyl carboxylic acids to 1,4-benzothiazines†
Abstract
A new ring expansion of 2-aminobenzothiazoles with alkynyl carboxylic acids was developed, which allows for one-pot synthesis of 1,4-benzothiazines in moderate to excellent yields. The cascade reaction was achieved through decarboxylative coupling, nucleophilic ring-opening reaction and intramolecular hydroamination process.
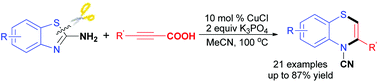

 Please wait while we load your content...
Please wait while we load your content...