Synthesis of (Z)-(arylamino)-pyrazolyl/isoxazolyl-2-propenones as tubulin targeting anticancer agents and apoptotic inducers†
Abstract
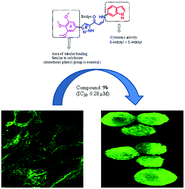
A new class of pyrazole and isoxazole conjugates were synthesized and evaluated for their cytotoxic activity against various human cancer cell lines. These compounds have shown significant cytotoxicity with lower IC50 values. FACS results revealed that A549 cells treated with these compounds arrested cells at the G2/M phase of the cell cycle apart from activating cyclin B1 protein levels. Particularly, compounds 9a and 9b demonstrated a remarkable inhibitory effect on tubulin polymerization and showed a pronounced inhibitory effect on tubulin polymerization with IC50 values of 1.28 μM and 0.28 μM respectively, whereas nocodazole, a positive control, has shown lower antitubulin activity with an IC50 value of 2.64 μM. Furthermore, these compounds induced apoptosis by loss of mitochondrial membrane potential, propidium iodide (PI) staining and the activation of caspase-3. Results of a fluorescence based competitive colchicine binding assay suggest that these conjugates bind successfully at the colchicine binding site of tubulin. These investigations reveal that such conjugates containing pyrazole with a trimethoxy phenyl ring and indole moieties have potential for the development of newer chemotherapeutic agents.

 Please wait while we load your content...
Please wait while we load your content...