A microwave-assisted multicomponent synthesis of substituted 3,4-dihydroquinazolinones†
Abstract
A microwave-assisted, multicomponent protocol for the synthesis of substituted 3,4-dihydroquinazolinones via a novel cascade imine/cyclization/aza-Henry reaction sequence is reported. Starting from o-formyl carbamates, a series of structurally diverse 3,4-dihydroquinazolinones was synthesized via a cyclic iminium ion intermediate in moderate to excellent yields. Notably, the reaction is fast, flexible, simple to perform and tolerates a variety of functional groups.
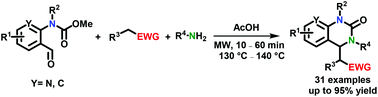
 Please wait while we load your content...
Please wait while we load your content...