Copper(ii)-catalyzed coupling reaction: an efficient and regioselective approach to N′,N′-diaryl acylhydrazines†
Abstract
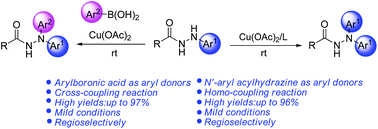
Using N′-aryl acylhydrazines as aryl donors, a novel copper(II)-catalyzed homo-coupling reaction of N′-aryl acylhydrazines has been developed for the synthesis of N′,N′-diaryl acylhydrazines. We also provided a complementary procedure for the preparation of unsymmetrical diaryl acylhydrazines via cross-coupling reaction. These protocols featured mild reaction conditions, wide functional group tolerance and highly regioselective products. Control experiments indicated that this kind of coupling reaction might undergo a transient acyl diazene intermediate.
 Please wait while we load your content...
Please wait while we load your content...