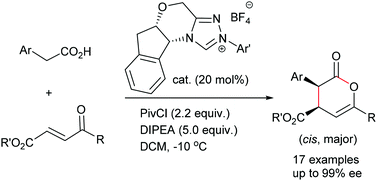
N-heterocyclic carbene-catalyzed cyclocondensation of 2-aryl carboxylic acids and enones: highly enantioselective synthesis of δ-lactones†
Abstract
The enantioselective N-heterocyclic carbene-catalyzed [4 + 2] cyclocondensation of 2-aryl carboxylic acids and enones was developed, affording the corresponding chiral δ-lactones in good yields with good diastereo- and high enantioselectivities.

 Please wait while we load your content...
Please wait while we load your content...