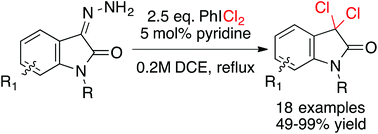
Synthesis of 3,3-dichloroindolin-2-ones from isatin-3-hydrazones and (dichloroiodo)benzene†
Abstract
Aryl- and N-substituted isatins were converted to isatin-3-hydrazones and subjected to a dichlorination reaction with PhICl2. Lewis base-catalysis was key to the reaction occurring rapidly and chemoselectively, providing 3,3-dichloroindolin-2-ones in 49–99% yield, and offering a new approach to the deoxygenative dihalogenation reaction.
 Please wait while we load your content...
Please wait while we load your content...