A mild copper-catalyzed aerobic oxidative thiocyanation of arylboronic acids with TMSNCS†
Abstract
A facile and efficient transformation of arylboronic acids to their corresponding aryl thiocyanates has been successfully developed. Based on the CuCl-catalyzed oxidative cross-coupling reaction between arylboronic acids and trimethylsilylisothiocyanate (TMSNCS) under oxygen atmosphere, the transformation can be readily conducted at ambient temperature. The newly-developed protocol provided a competitive synthetic approach to aryl thiocyanates that can tolerate a broad range of reactive functional groups and/or strong electron-withdrawing groups.
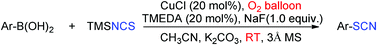
 Please wait while we load your content...
Please wait while we load your content...