Interactions of arene ruthenium metallaprisms with human proteins†
Abstract
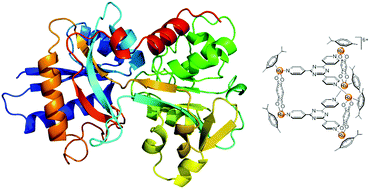
Interactions between three hexacationic arene ruthenium metallaprisms, [(p-cymene)6Ru6(tpt)2(dhnq)3]6+, [(p-cymene)6Ru6(tpt)2(dhbq)3]6+ and [(p-cymene)6Ru6(tpt)2(oxa)3]6+, and a series of human proteins including human serum albumin, transferrin, cytochrome c, myoglobin and ubiquitin have been studied using NMR spectroscopy, mass spectrometry and circular dichroism spectroscopy. All data suggest that no covalent adducts are formed between the proteins and the metallaprisms. Indeed, in most cases electrostatic interactions, leading to precipitation of protein-metallaprism aggregates, have been observed. In addition, with the smallest proteins, ubiquitin, myoglobin and cytochrome c, the presence of the hexacationic arene ruthenium metallaprisms induces structural changes of the proteins, as emphasized by circular dichroism. The results suggest that proteins are certainly a biological target for these metalla-assemblies.
- This article is part of the themed collection: Supramolecular Chemistry in Water
 Please wait while we load your content...
Please wait while we load your content...