Synthesis of labile all-trans-7,8,7′,8′-bis-acetylenic carotenoids by bi-directional Horner–Wadsworth–Emmons condensation†
Abstract
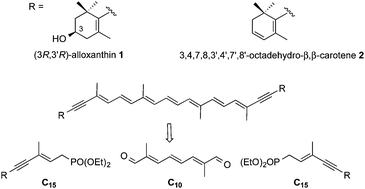
A new stereoselective synthesis of the C40-bis-acetylenic carotenoids all-trans-(3R,3′R)-alloxanthin and all-trans-3,4,7,8,3′,4′,7′,8′-octadehydro-β,β-carotene, both compounds featuring a stereochemically labile C7–C10 enyne, based on a bi-directional Horner–Wadsworth–Emmons (HWE) reaction of a C15-phosphonate and a central C10-dialdehyde, is reported. The triene unit of the latter fragment was synthesized using the acyclic metathesis/dimerization reaction.

 Please wait while we load your content...
Please wait while we load your content...