Mechanisms of imine exchange reactions in organic solvents
Abstract
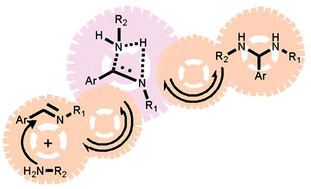
The state of the art in the mechanisms operating in imine chemistry in organic solvents is critically discussed in the present review. In particular, the reaction pathways involved in imine formation, transimination and imine metathesis in organic media are taken into account, with the aim of organizing the poor, and sometimes scattered, information available in the literature. It is shown that 4-membered cyclic transition states, either polar or apolar, can be considered a leitmotif for the chemistry of imines in organic solvents. However, it is pointed out that further investigations will be necessary to reach an adequate degree of knowledge of the mechanisms involved in such important reversible processes.
 Please wait while we load your content...
Please wait while we load your content...