Selective detection of Al3+ and citric acid with a fluorescent amphiphile†
Abstract
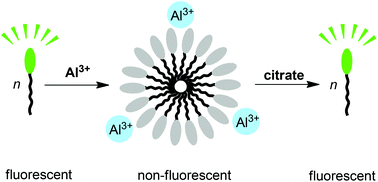
An amphiphilic fluorescent dye with a disulfonated BODIPY head group and a heptadecyl side chain is described. In buffered aqueous solution, the amphiphile can form aggregates with a critical micelle concentration of ∼20 μM. The aggregation of the dye is associated with a strong quenching of its fluorescence. Al3+ promotes aggregation, whereas other metal ions have a much smaller effect, in particular when histidine is added as masking agent. The Al3+-induced aggregation can be used to sense Al3+ in the low micromolar concentration range with high selectivity. Furthermore, we demonstrate that a dye–Al3+ mixture can be used as a sensing ensemble for the detection of citric acid. The assay allows quantifying the citric acid content of commercial beverages such as energy drinks.
- This article is part of the themed collection: Supramolecular Chemistry in Water

 Please wait while we load your content...
Please wait while we load your content...