UV-visible and 1H–15N NMR spectroscopic studies of colorimetric thiosemicarbazide anion sensors†
Abstract
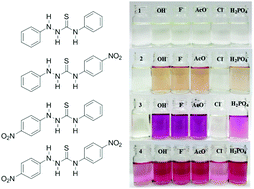
Four model thiosemicarbazide anion chemosensors containing three N–H bonds, substituted with phenyl and/or 4-nitrophenyl units, were synthesised and studied for their anion binding abilities with hydroxide, fluoride, acetate, dihydrogen phosphate and chloride. The anion binding properties were studied in DMSO and 9 : 1 DMSO–H2O by UV-visible absorption and 1H/13C/15N NMR spectroscopic techniques and corroborated with DFT studies. Significant changes were observed in the UV-visible absorption spectra with all anions, except for chloride, accompanied by dramatic colour changes visible to the naked eye. These changes were determined to be due to the deprotonation of the central N–H proton and not due to hydrogen bonding based on 1H/15N NMR titration studies with acetate in DMSO-d6–0.5% water. Direct evidence for deprotonation was confirmed by the disappearance of the central thiourea proton and the formation of acetic acid. DFT and charge distribution calculations suggest that for all four compounds the central N–H proton is the most acidic. Hence, the anion chemosensors operate by a deprotonation mechanism of the central N–H proton rather than by hydrogen bonding as is often reported.
- This article is part of the themed collection: Supramolecular Chemistry in Water

 Please wait while we load your content...
Please wait while we load your content...