Aryl-bis-(scorpiand)-aza receptors differentiate between nucleotide monophosphates by a combination of aromatic, hydrogen bond and electrostatic interactions†
Abstract
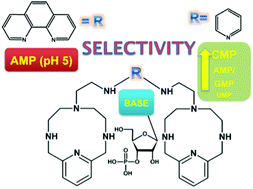
Bis-polyaza pyridinophane scorpiands bind nucleotides in aqueous medium with 10–100 micromolar affinity, predominantly by electrostatic interactions between nucleotide phosphates and protonated aliphatic amines and assisted by aromatic stacking interactions. The pyridine-scorpiand receptor showed rare selectivity toward CMP with respect to other nucleotides, whereby two orders of magnitude affinity difference between CMP and UMP was the most appealing. The phenanthroline-scorpiand receptor revealed at pH 5 strong selectivity toward AMP with respect to other NMPs, based on the protonation of adenine heterocyclic N1. The results stress that the efficient recognition of small biomolecules within scorpiand-like receptors relies mostly on the electrostatic and H-bonding interactions despite the competitive interactions in the bulk solvent, thus supporting further optimisation of this versatile artificial moiety.
- This article is part of the themed collection: Supramolecular Chemistry in Water

 Please wait while we load your content...
Please wait while we load your content...