Design and synthesis of fluorescent 7-deazaadenosine nucleosides containing π-extended diarylacetylene motifs†
Abstract
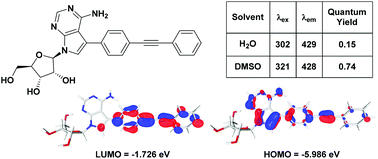
C-modified 7-deazaadenosines containing a diphenylacetylene moiety have been synthesised using cross-coupling approaches. The C-modified nucleosides exhibit remarkable fluorescence properties, including high quantum yields. Solvatochromic studies show a near linear correlation between the Stokes shift and solvent polarity which is indicative of intramolecular charge transfer. DFT calculations have allowed us to correlate the experimentally observed photophysical properties with the calculated HOMO–LUMO energy gaps within a series of real and model compounds.


 Please wait while we load your content...
Please wait while we load your content...