Artificial metalloenzymes for the diastereoselective reduction of NAD+ to NAD2H†
Abstract
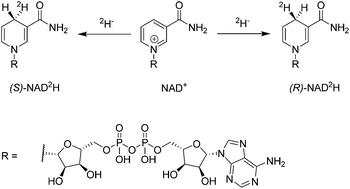
Stereoselectively labelled isotopomers of NAD(P)H are highly relevant for mechanistic studies of enzymes which utilize them as redox equivalents. Whereas several methods are firmly established for their generation in high diastereomeric purity by enzymatic methods, alternative methods have so far not been investigated. The article presents the stereoselective deuteration of NAD+ at the 4-position (90% de) of the pyridinium-ring by means of an artificial metalloenzyme. The artificial metalloenzyme consists of a biotinylated iridium cofactor embedded in streptavidin isoforms and the resulting constructs have been previously shown to be compatible with natural enzymes. Alternative methods for stereoselective NAD(P)+ reduction are expected to be of high interest for the mechanistic study of enzymes that accept NAD(P)H mimics and for the synthesis of structurally related fine chemicals.
- This article is part of the themed collection: Supramolecular Chemistry in Water

 Please wait while we load your content...
Please wait while we load your content...