Kinetic evaluation of glucose 1-phosphate analogues with a thymidylyltransferase using a continuous coupled enzyme assay†
Abstract
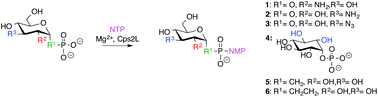
Cps2L, a thymidylytransferase, is the first enzyme in Streptococcus pneumoniaeL-rhamnose biosynthesis and an antibacterial target. We herein report the evaluation of six sugar phosphate analogues selected to further probe Cps2L substrate tolerance. A modified continuous spectrophotometric assay was employed for facile detection of pyrophosphate (PPi) released from nucleotidylyltransfase-catalysed condensation of sugar 1-phosphates and nucleoside triphosphates to produce sugar nucleotides. Additionally, experiments using waterLOGSY NMR spectroscopy were investigated as a complimentary method to evaluate binding affinity to Cps2L.
 Please wait while we load your content...
Please wait while we load your content...