Copper(i)-promoted cycloalkylation–peroxidation of unactivated alkenes via sp3 C–H functionalisation†
Abstract
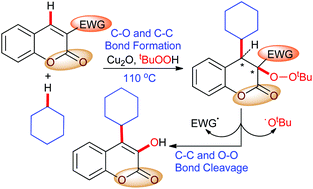
A copper(I)-promoted cycloalkylation–peroxidation strategy has been developed via a three-component reaction involving cycloalkanes, tert-butyl hydroperoxide (TBHP) and internal conjugated alkenes possessing electron-withdrawing groups (EWGs). This process installs C–O and C–C bonds via sp3 C–H functionalisation with concomitant generation of two stereocentres. This regioselective radical addition of coumarin system is opposite to that of styrene.

 Please wait while we load your content...
Please wait while we load your content...