Synthesis of novel polyhydroxylated pyrrolidine–triazole/-isoxazole hybrid molecules†
Abstract
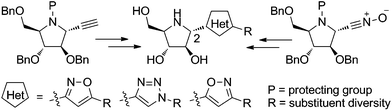
A straightforward synthesis of novel, 2-heterocyclyl polyhydroxylated pyrrolidines is described. Stereocontrolled additions of nucleophiles to cyclic nitrones generated the corresponding 2,3-trans adducts, allowing the synthesis of the corresponding pyrrolidines via key intermediates bearing an alkyne and a nitrile oxide. Three hybrid systems, including a pyrrolidine with two isoxazoles and one triazole, are efficiently prepared via 1,3-dipolar cycloaddition. Biological testing of the product alkaloids showed that subtle structural variations have drastic effects on their inhibitory activities against glucosidases.
 Please wait while we load your content...
Please wait while we load your content...