A QSAR study on the inhibition mechanism of matrix metalloproteinase-12 by arylsulfone analogs based on molecular orbital calculations†
Abstract
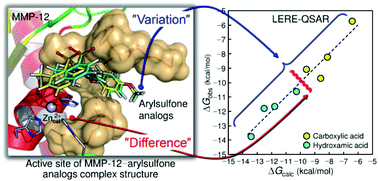
A binding mechanism between human matrix metalloproteinase-12 (MMP-12) and eight arylsulfone analogs having two types of carboxylic and hydroxamic acids as the most representative zinc binding group is investigated using a quantitative structure–activity relationship (QSAR) analysis based on a linear expression by representative energy terms (LERE). The LERE–QSAR analysis quantitatively reveals that the variation in the observed (experimental) inhibitory potency among the arylsulfone analogs is decisively governed by those in the intrinsic binding and dispersion interaction energies. The results show that the LERE–QSAR analysis not only can excellently reproduce the observed overall free-energy change but also can determine the contributions of representative free-energy changes. An inter-fragment interaction energy difference (IFIED) analysis based on the fragment molecular orbital (FMO) method (FMO–IFIED) leads to the identification of key residues governing the variation in the inhibitory potency as well as to the understanding of the difference between the interactions of the carboxylic and hydroxamic acid zinc binding groups. The current results that have led to the optimization of the inhibitory potency of arylsulfone analogs toward MMP-12 to be used in the treatment of chronic obstructive pulmonary disease may be useful for the development of a new potent MMP-12 inhibitor.
 Please wait while we load your content...
Please wait while we load your content...