Exploiting the narrow gap of rearrangement between the substituents in the vicinal disubstitution reactions of diaryliodonium salts with pyridine N-sulfonamidates†
Abstract
The vicinal disubstitution reactions of diaryliodonium salts with pyridine N-sulfonamidates to give o-pyridinium anilines were fully examined. A reaction pathway of N-arylation occurring at the amidate group followed by a radical rearrangement is proposed. The electronic effects of various substituents in this radical rearrangement were investigated.
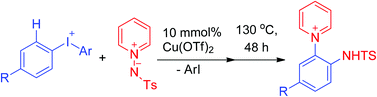
 Please wait while we load your content...
Please wait while we load your content...