Catalytic asymmetric desymmetrization approaches to enantioenriched cyclopentanes
Abstract
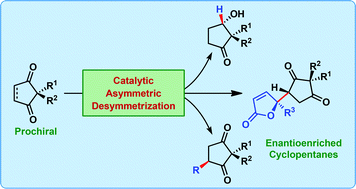
Catalytic asymmetric desymmetrization represents an excellent strategy for accessing highly functionalized chiral building blocks. However, the application of desymmetrization for the synthesis of enantioenriched cyclopentane derivatives remained limited, when compared to chiral cyclohexanes. We have recently developed a desymmetrization protocol for prochiral 2,2-disubstituted cyclopentene-1,3-diones by direct catalytic asymmetric vinylogous nucleophilic addition of deconjugated butenolides. In this perspective, we give an overview of asymmetric desymmetrization reactions leading to enantioenriched cyclopentanes and their derivatives. The focus is kept confined to the diverse nature of reactions used for this purpose. A brief discussion on the potential future directions is also provided.

 Please wait while we load your content...
Please wait while we load your content...