Polyketide synthase and non-ribosomal peptide synthetase thioesterase selectivity: logic gate or a victim of fate?†
Abstract
Covering: 1999 to 2014
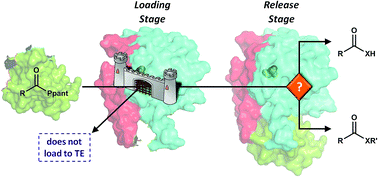
Type 1, α/β hydrolase-like thioesterase (TE) domains are essential offloading enzymes, releasing covalently bound products from fatty acid, polyketide, and non-ribosomal peptide biosynthetic complexes. The release step can occur by attack of an exogenous nucleophile effecting hydrolysis or transesterification or by an intramolecular O-, N-, or C-nucleophile, effecting macrolactonization, macrolactamization or Claisen-like condensation of the product. Thus in addition to ensuring turnover of the pathway, TEs provide access to increased chemical diversity. We review the diversity, structure, and mechanism of PKS and NRPS TEs and discuss recent works that highlight the role of TEs as potential arbitrators in offloading. In particular, we examine cases where TEs act as logic gates that ask a particular question about the substrate and use this information to determine the substrate's fate. As the TE mechanism occurs via two steps, we analyze both the loading and release steps independently as logic gates. The use of logic gates provides an important perspective when evaluating the evolution of TEs within a pathway, as well as highlighting work towards the goal of predicting TE function in unknown and engineered pathways.
- This article is part of the themed collection: Biosynthetic Assembly Lines

 Please wait while we load your content...
Please wait while we load your content...