Ionic liquids as solvents for the Knoevenagel condensation: understanding the role of solvent–solute interactions†
Abstract
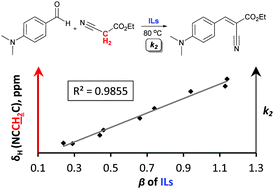
The Knoevenagel condensation in ionic liquids has been demonstrated as a strongly solvent-dependent process. The objective of this study was to establish a simple and descriptive trend of solvent–solute interactions that favour the Knoevenagel condensation in ionic liquid media. The reaction between 4-(dimethylamino)benzaldehyde and ethyl cyanoacetate in several imidazolium- and pyrrolidinium-based ionic liquids and one ionic liquid mixture was studied. The rate constants were rationalised by studying the change in the 1H NMR chemical shift of representative starting materials in the ionic liquids and the measurement and consideration of the Kamlet–Taft descriptors for each solvent. The hydrogen bond basicity (β) of ionic liquids was confirmed to be the dominant solvent descriptor in determining the rate of the Knoevenagel condensation. Therefore, the descriptors obtained have been demonstrated as useful guidelines for predicting the optimal choice of an ionic liquid for the promotion of the Knoevenagel condensation.

 Please wait while we load your content...
Please wait while we load your content...