Electronic and structural properties of polymers based on phenylene vinylene and thiophene units. Control of the gap by gradual increases of thiophene moieties†
Abstract
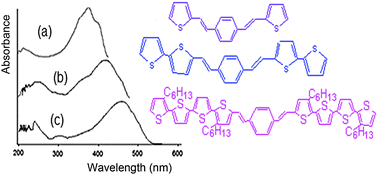
Several poly(thiophene) derivatives containing p-phenylenevinylene (PV) as an electron-donor were synthesized using FeCl3. PV units are regularly spaced in two, four and eight thiophenyl units across the main chain. PV units and the gradual increase in the number of thiophene units affect the properties of polymers such as poly(3-hexylthiophene), (P3HT). Polymers, labeled as poly(FV1Th), poly(FVBiTh) and poly(FVTeTh) were characterized by FT-IR and UV-Vis spectrometry, elemental analysis, thermal stability (TGA), differential scanning calorimetry (DSC) and cyclic voltammetry (CV). Conjugation increases with increasing thiophene rings in the main chain and the conjugation is higher in polymers that exhibited different optical absorption, effective conjugation, similar intrinsic viscosity, and high thermal stability with a weight loss less than 10% at decomposition temperatures higher than 300 °C (except poly(FVTeTh). DSC showed melting points over 200 °C and the formation of crystalline zones was found in all cases. An increase in the number of thiophene rings in the main chain resulted in a more ordered crystalline molecular structure. Moreover, the polymers presented redox processes at a potential lower than that of P3HT. The Highest Occupied Molecular Orbital (HOMO), Lowest Unoccupied Molecular Orbital (LUMO) and optical band gap (Eg) were measured and the values obtained were compared with those of P3HT. The effect caused by PV units and the increase of thiophenyl units in the chains on HOMO, LUMO, band gap, fusion, crystallinity and TGA is reported. The Eg of the monomers was greater than that of P3HT. The results showed that with respect to P3HT, polymers HOMO and LUMO are lower and in some cases by a slight change in Eg. The PV effect as an electron donor causes a decrease in the HOMO and LUMO, envisaging them as potential polymers to be studied in organic photocells.

 Please wait while we load your content...
Please wait while we load your content...