Gold oxyfluorides, Au(OF)n (n = 1–6): novel superhalogens with oxyfluoride ligands†
Abstract
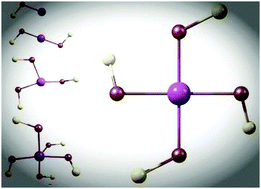
Superhalogen species, having the electron affinities higher than that of halogens, play a consequential role in the design of unusual compounds possessing high oxidizing capabilities. Traditionally, such species include a metallic or nonmetallic core with electronegative atoms (F, Cl, and O) or moieties (CN, NCO, and SCN). Note that the electron affinities of these moieties are comparable to or even larger that of F. In the quest for other ligands that might be capable to induce the superhalogen behavior in metal complexes, we have investigated the OF moiety, whose electron affinity is lower than that of F. Utilizing gradient corrected density functional theory, we have systematically studied Au(OF)n complexes (n = 1–6) and their anions. The adiabatic electron affinities of Au(OF)n and vertical detachment energies of Au(OF)n− suggest that these species behave as superhalogens for n ≥ 2. The electron affinities of Au(OF)n, which are smaller than AuFn and Au(CN)n complexes but comparable to that of AuCln, are explained on the basis of extra electron delocalization over several OF moieties. Unlike Au(CN)n (n ≥ 2), which become unstable against dimerization of CN moieties due to their large binding energies, Au(OF)n (n ≤ 4) and Au(OF)n− (n ≤ 5) are found to be stable. The enhanced stability of Au(OF)2− and Au(OF)4− is particularly highlighted. Therefore, the OF moiety is capable of stabilizing the oxidation state of Au up to +4. Note that the highest possible oxidation state of Au is limited to +5. Thus, the investigation advocates that the OF moiety performs better than CN for the stabilization of metal complexes in higher oxidation states. However, the electron affinities of metal complexes stabilized with OF are lower than those with F ligands in accordance with the electronic structure and relatively lower electron affinity of OF.


 Please wait while we load your content...
Please wait while we load your content...