Structural, vibrational and electronic characterization of 1-benzyl-3-furoyl-1-phenylthiourea: an experimental and theoretical study†
Abstract
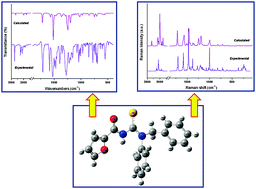
1-Benzyl-3-furoyl-1-phenylthiourea is a thiourea derivative synthesized and characterized by means of vibrational spectroscopy (IR and Raman) multinuclear NMR (1H and 13C) and elemental analysis. The geometrical parameters of this compound obtained from XRD studies were compared with the calculated values [B3LYP/6-311++G(d,p)] showing a good agreement. As determined by XRD analysis performed previously, the title compound exhibits the U-shape conformation with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S double bonds in anticlinal geometry. This conformational feature is mainly dictated by the substitution degree on the thiourea core and the ability to form an intramolecular N–H⋯O
S double bonds in anticlinal geometry. This conformational feature is mainly dictated by the substitution degree on the thiourea core and the ability to form an intramolecular N–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C hydrogen bond. The UV-visible absorption spectra of the compound in methanol solution were recorded and analyzed using time dependent density functional theory (TD-DFT). Molecular stability was investigated by applying the natural bond (NBO) analysis. Intermolecular interactions were evaluated by means of the AIM approach. The calculated HOMO and LUMO energies show that the charge transfer occurs in the molecule. The molecular electrostatic potential map was calculated by the DFT method. Non-linear optical (NLO) behavior of the title compound was investigated by determining the electric dipole moment, polarizability α, and hyperpolarizability β using B3LYP/6-311++G(d,p) approximation.
C hydrogen bond. The UV-visible absorption spectra of the compound in methanol solution were recorded and analyzed using time dependent density functional theory (TD-DFT). Molecular stability was investigated by applying the natural bond (NBO) analysis. Intermolecular interactions were evaluated by means of the AIM approach. The calculated HOMO and LUMO energies show that the charge transfer occurs in the molecule. The molecular electrostatic potential map was calculated by the DFT method. Non-linear optical (NLO) behavior of the title compound was investigated by determining the electric dipole moment, polarizability α, and hyperpolarizability β using B3LYP/6-311++G(d,p) approximation.

 Please wait while we load your content...
Please wait while we load your content...