Synthesis of Bi2Sn2O7 and enhanced photocatalytic activity of Bi2Sn2O7 hybridized with C3N4†
Abstract
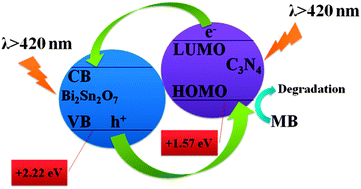
Bi2Sn2O7 photocatalysts were synthesized by a hydrothermal method. Bi2Sn2O7 photocatalysts showed highly efficient photocatalytic activity for the degradation of methylene blue under visible light irradiation. Kinetic studies using radical scavenger technologies suggested that holes were the dominant photooxidants. After hybridization with C3N4, the photocatalytic activity of Bi2Sn2O7 was obviously enhanced. The enhanced photocatalytic activity of the C3N4/Bi2Sn2O7 photocatalysts could be attributed to the effective separation of the photogenerated e−/h+ pairs. The photogenerated holes on the valence band of Bi2Sn2O7 can transfer to the highest occupied molecular orbital of C3N4via the well developed interface, causing a reduction in the probability of e−/h+ recombination; consequently, large numbers of photogenerated holes led to enhancing the photocatalytic activity.

 Please wait while we load your content...
Please wait while we load your content...