Reactivity of halfsandwich rare-earth metal methylaluminates toward potassium (2,4,6-tri-tert-butylphenyl)amide and 1-adamantylamine†
Abstract
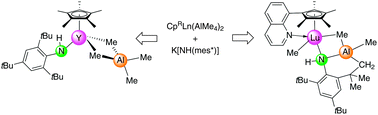
The equimolar reaction of potassium (2,4,6-tri-tert-butylphenyl)amide with Cp*Ln(AlMe4)2 (Cp* = 1,2,3,4,5-pentamethyl cyclopentadienyl) yielded {Cp*Ln(AlMe4)[NH(mes*)]}x (Ln = Y, La; mes* = C6H2tBu3-2,4,6). The treatment of Cp*Ln(AlMe4)[NH(mes*)] with tetrahydrofuran led to intramolecular C–H bond activation of a tBu group with the formation of Cp*YMe{NH[C6H2tBu2-2,4-(CMe2CH2)-6]}(AlMe2)(thf). A similar methyl-anilide species CpQLuMe{NH[C6H2tBu2-2,4-(CMe2CH2)-6]}(AlMe2) (CpQ = 2,3,4,5-tetramethyl-1-(8-quinolyl)cyclopentadienyl) with a C–H bond activated ligand backbone formed by the reaction of CpQLu(AlMe4)2 and K[NH(mes*)]. The reactivity of CpQY(AlMe4)2 toward H2NAd (Ad = adamantyl) ultimately led to the methyl–amide complex CpQYMe[NH(Ad)](AlMe3), corroborating the presence of competing deprotonation and donor-induced methylaluminate cleavage reactions. The halfsandwich complexes CpQLu(AlMe4)2, Cp*Y(AlMe4)[NH(mes*)], Cp*YMe{NH[C6H2tBu2-2,4-(CMe2CH2)-6]}(AlMe2)(thf), CpQLuMe{NH[C6H2tBu2-2,4-(CMe2CH2)-6]}(AlMe2), and CpQYMe[NH(Ad)](AlMe3) as well as the side-product AlMe3(H2NAd) were fully characterized by NMR/FTIR spectroscopy, elemental analysis, and X-ray crystallography.
- This article is part of the themed collection: Frontiers of Organo-f-Element Chemistry

 Please wait while we load your content...
Please wait while we load your content...