Sulfur difluoride and sulfur monofluoride as ligands in iron carbonyl chemistry†
Abstract
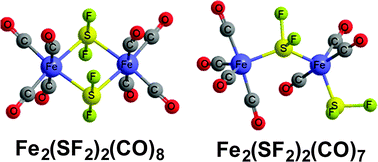
Density functional theory predicts a binuclear Fe2(μ-SF2)2(CO)8 octacarbonyl structure with two bridging SF2 groups and a long Fe⋯Fe distance of ∼3.5 Å indicating a lack of a direct iron–iron bond. In addition, three Fe2(μ-SF2)(SF2)(CO)7 stereoisomers of similar energies are found with one bridging SF2 group and one terminal SF2 group and an even longer Fe⋯Fe distance of ∼3.9 Å likewise indicating a lack of a direct iron–iron bond. In contrast to the binuclear Fe2(SF2)2(CO)n (n = 8, 7) derivatives, the mononuclear Fe(SF2)(CO)n (n = 4, 3) are disfavored by ∼10 kcal mol−1 for n = 4 to ∼30 kcal mol−1 for n = 3, respectively, with respect to fluorine shift from sulfur to iron to give the corresponding Fe(SF)(F)(CO)n derivatives. The SF ligands in the tetracarbonyls Fe(SF)(F)(CO)4 are one-electron donor ligands with Fe–S distances of ∼2.3 Å. However, the SF ligands in the tricarbonyls Fe(SF)(F)(CO)3 are three-electron donor ligands with significantly shorter Fe![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S distances of ∼2.1 Å.
S distances of ∼2.1 Å.

 Please wait while we load your content...
Please wait while we load your content...