4-Aminoquinoline-pyrimidine-aminoalkanols: synthesis, in vitro antimalarial activity, docking studies and ADME predictions†‡
Abstract
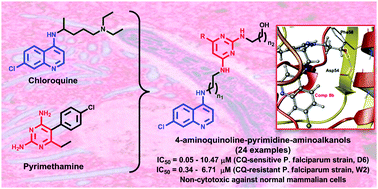
Twenty-four new 4-aminoquinoline-pyrimidine hybrids containing a terminal aliphatic amino-alcohol chain were synthesized and assessed for their antimalarial activity against chloroquine-sensitive (D6) and chloroquine-resistant (W2) strains of Plasmodium falciparum. All of the compounds displayed potent antiplasmodial activities (IC50 values in the range of 0.05–10.47 μM) with no appreciable cytotoxicity towards mammalian cells, up to the highest tested concentration of 12 μM. Molecular docking studies of the most active compounds (8b–8f, 8u and 8v) with both wild type and quadruple mutant Pf-DHFR-TS were performed, which exhibited interactions comparable to conventional folate inhibitors. ADME predictions also revealed favourable pharmacokinetic parameters for the synthesized hybrids, which warrants their suitability for development as potent antimalarials.

 Please wait while we load your content...
Please wait while we load your content...